The
discovery of neutron by Chadwick in 1932 set up a new field of transmutation
reaction by using the neutrons as projectiles. In this line, the work from
three groups of scientist: (i) Enrico Fermi in Italy, (ii) F. Joliot Curie and Savitch
in France, and (iii) Otto Hahn and Strassman in Germany, paved the way
(1934-1938) in discovering the nuclear fission.
In
1936, E. Fermi suggested that the compound nucleus (236U) formed in
the bombardment of slow neutrons on 235U92 should undergo
β- decay because of a higher neutron to proton ratio in the compound
nucleus. This suggestion was tested in laboratory and it showed β-
activity with four different half-lives. Considering four successive β-
decays in the daughter nuclei, there should be four transuranic elements having
Z = 93, 94, 95 and 96. The group of J. Curie carried out chemical analysis on
the uranium compounds bombarded by the slow neutrons. Astonishingly, they
noticed a chemical species having properties close to those of Z = 56-67 far
away from Z = 92 in the periodic table. They noticed this surprising
observation but did not confirm the observation. Thus the credit of the
discovery of nuclear fission slipped away From their hands to the group of Otto
Hahn who established barium (Z = 56) as one of the product in the 235U92
+ n reactions. Thus the barium produced showed β- activity. In
the analysis, the production of Krypton (Z = 36) was also proved. Thus, product
nuclides were found to be much lighter than the starting nuclide. This is why,
it was suggested by Meinter (a former associate of Hahn) and Firsch that the
uranium nucleus on being bombarded by a slow neutron, splits into two lighter
fragments of comparable size and the process was termed as nuclear fission.
So,
we can say that nuclear fission is a nuclear reaction in which the nucleus of
an atom splits into two or more smaller nuclei, typically accompanied by the
release of a large amount of energy.
235U92
+ 1n0 (slow) → 236U92 → 141Ba56
+ 92Kr36 + 3(1n0) + energy
Characteristic
Features of Nuclear Fission
a) Mass
distribution in fission fragments:
The compound nucleus formed may undergo fission in a variety of ways. Some
possibilities are given below:
Thus,
the compound nucleus (236U*) very often undergoes an asymmetric
fission in about 30 routes. The mass number of the lighter fission product lies
in the range 85 to 104, while the heavier fragment covers the range, 130 to
149. The most probable fission path involves the fragments with mass numbers
around 95 to 139. The distribution of mass number can be represented in the
fission yield curve known as Bohr yield curve.
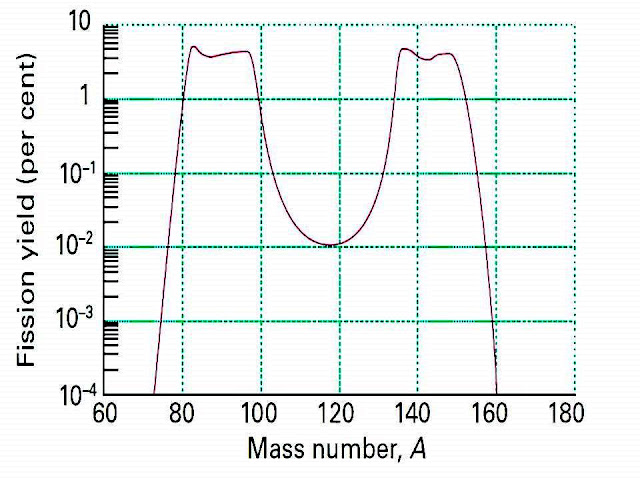 |
| Fission yield curve (known as Bohr yield curve) |
The
symmetric fission producing the fragments with mass number -117 is a rare
possibility and it is evident. However, it has been proved that with the
increase of energy of the projectile neutron affecting the fission, the
probability of symmetric fission increases. It is experimentally verified in
239Pu.
b) Energy
distribution in fission fragments:
The mass distribution pattern is intimately related with the energy
distribution pattern among the fission products. If the compound nucleus
undergoing fission is assumed to be at rest, and the energies shared by the
emitted neutrons are neglected, then according to the law of conservation of
momentum, it gives,
M1u1
= M2u2
Or,
u1/u2 = M2/M1
Where
M1 and M2 denote masses of the two fragments, and u1
and u2 represent their corresponding velocities. Therefore, for
kinetic energies
E1/E2
= [½ M1u12]/[ ½ M2u22]
= M2/M1 (as M1u1 = M2u2)
Thus,
the masses and kinetic energies of the fragments are inversely related. The
distribution of kinetic energy among the fission products can be measured by
using an ionisation chamber.
c)
Emission of projectiles: The
projectiles affecting the nuclear fission are emitted (2-3 per fission) in the
process. Thus, the ejectiles under a suitable condition can be reutilized as
projectiles to make the process self-sustained.
Here,
it is worth mentioning that all the neutrons are not emitted at one instant.
More than 99% are emitted almost instantaneously in a time of the order of 10-14
s. These neutrons are called prompt neutrons. The neutrons (1%) which are
emitted in late from the fission products are called delayed neutrons.
Prompt
neutrons are released within 10-14 s from the neutron-rich fragments
of fission products. Thus the fission of 235U by a thermal neutron
can be represented as:
235U92
+ 1n0 → (236U92*) → 146La57
+ 87Br35 + 3(1n0) (prompt neutrons)
Here,
87Br is a β- emitter and it decays with a half-life of
55.5 s to 87Kr which is sufficient energy to eject a neutron
immediately to produce the stable nuclide 86Kr, i.e.
87Br
→ 87Kr* + β- → 86Kr + n (delayed neutron)
Here,
the neutron emission rate is determined by the slow step (t1/2 =
55.5 s). Such neutrons, emitted after a measurable time period of the fission
are described as delayed neutrons which play an important role in controlling
the nuclear fission in nuclear reactors. The delayed neutrons are of low energy
while the prompt neutrons are of high energy.
d) β- Activity
in the fission products:
In the products fragments, the neutron to proton ratio lies far above their
corresponding stability region. The starting nuclide (Z = 92) requires a
relatively higher neutron to proton ratio for its stability and this high ratio
on being transmitted to the fission products of much lower atomic number makes
them unstable with respect to β- activity. The process goes on
until the stable nuclei are formed. Thus in a particular fission reaction, two
different series of isobaric nuclides with the mass number A1 and A2
(where the initial fragments are characterized with the mass number A1
and A2) are obtained. The β- activities of some fission
products are shown below.
e)
Energy (Q) release in fission: The
energy released per fission is ≈ 200 MeV which is much greater than that
released in any ordinary nuclear reaction. Due to the ejection of the
projectiles, once the process is started, it can process in a chain reaction to
release an enormous amount of energy in a moment. The calculation of energy
release in the process can be done in two ways as discussed below.
i) Mass defect method:
For
this purpose, let us consider one of the most probable fission reactions, i.e.
235U92
+ 1n0 → 98Mo42 + 136Xe54
+ 2(1n0) + 4 (0e-1) + 4 (0ν0)
Here,
mass loss Δm ≈ [ m(235U) + m(1n)] – [m(98Mo) +
m(136Xe) + 2m(1n)]
=
(235.044 + 1.0086) – (97.906 + 135.907 + 2×1.0086)
=
0.2224 amu or (0.2224×931) MeV = 207 MeV
In
calculating Δm, the mass of the emitted electrons has been neglected.
ii) Nuclear binding
energy (NBE) method:
For
the nuclear binding energy curves, it is evident that for the fissionable
nuclides (A ≈ 235 – 240), the binding energy per nucleon lies in the range ~
7.5 MeV while the fission products stand on the curve where B ~ 8.5 MeV. Thus
in moving from the position of the fissionable nuclide to that of the fission
products, ~1 (= 8.5-7.5) MeV energy is released per nucleon. Thus in each
fission, ~235 × 1 = 235 MeV energy is released (neglecting the neutrons emitted
as ejectiles).
Reference
1) Concise inorganic chemistry by J. D. Lee.
2) Inorganic Chemistry by James E. Huheey, Ellen A Keither, Richard L. Keither, Okhil K. Medhi.



Comments
Post a Comment