Pinacols
are ditertiary 1, 2- diols. The simplest member of the class is Me2C(OH).C(OH)Me2.
When this treated with dilute or moderately concentrated H2SO4,
a rearrangement reaction takes place which lead to the formation of Me3CCOMe
(pinacolone), and the rearrangement is known as the pinacol-pinacolone
rearrangement.
Nowadays
the acid-catalysed rearrangement reactions of 1, 2-diols to oxo compounds,
aldehydes or ketones, are called the pinacol-pinacolone rearrangements. For
example
These
examples show that the migration origin and migration terminus are the two
adjacent C atoms, and the migrating group may be an aryl or an alkyl group, or
an H atom etc. Migration of a bond may also occur in this rearrangement whereby
ring expansion and ring contraction reactions may take place.
Rearrangement
reactions of 1, 2-halohydrines and 1, 2-amino-alchohols to pinacolones are
analogous reactions and called the pinacolic rearrangements; these are carried
out by treating the former compounds with Ag+ and the latter
compounds with HNO2 (NaNO2/HCl).
Since highly
branched oxo compounds are very difficult to prepare by the other reactions,
this rearrangement has interesting applications in synthesis.
The diol
F3CPhC(OH)C(OH)MeCF3 containing strong
electron-withdrawing –CF3 group does not undergo pinacol
rearrangement and this is because the –CF3 group highly disfavors
the formation of a carbocation. In fact, by intensifying the positive charge,
it destabilizes the carbocation and thereby increases the energy of activation
of the rate-determining step.
Step 1:
Reversible protonation to a hydroxyl group and the elimination of water
molecule; an electron-deficient carbenium ion is thus formed in this step:
Step 2:
The formation of a non-classical carbenium ion, a bridged intermediate. Here it
is important to note that the migrating group never detaches itself from the
substrate skeleton and thus the reaction becomes an intramolecular one. When an
aryl group migrates, the bridged intermediate is an aryl cation which may be an
actual compound since it is reasonably stable.
Since the mechanism involves the migration of a group with its bonded electrons to an adjacent carbon atom, it follows the mechanism of anionotropic 1, 2-shift. It has been known from the Kinetic study that the step in which elimination of water molecule occurs from the protonated 1, 2 -diol is the slow step and hence it is the rate-determining step. The intramolecular nature of the reaction is supported by the crossover experiment in which a mixture of Ph2COH.COHMe2 and Ph2COH.COHEt2 has been treated with acid. In this reaction, only intramolecular products, i.e., Ph2CMe.COMe and Ph2CEt.COEt, have been obtained; Ph2CEt.COMe and Ph2CMeCOEt, the possible cross products, have not yet been isolated. Thus cross migration does not take place in this reaction. This shows that the reaction is essentially and intramolecular one.
The
evidence for the intermediacy of carbocation in this reaction is following:
When
pinacol is treated with acid in H218O solution, the
recovered pinacol is found to contain the 18O without the structure
being rearranged. This observation suggests that this rearrangement involves
reversible formation of carbocation.
When
pinacol rearrangement involving a hydride shift is carried out in D2O,
no deuterium is found to be incorporated in the final rearranged product. This
observation also suggests that the rearrangement is strictly intramolecular.
Now the
question is which -OH groups will get protonated and leave and which of the two
groups will migrate from the migration origin.
Any of
the two -OH groups may leave from the symmetrical pinacol RR'C(OH)C(OH)RR'
since the same carbocation is formed no matter which OH leaves. However, the
-OH that lives from the unsymmetrical pinacol R2C(OH)C(OH)R'2
is the one whose loss gives rise to the more stable carbocation. For example 1,
1 - diphenylethanediol gives diphenylacetaldehyde, not diphenylacetophenone and
this is because Ph2C+CH2OH is far more stable than Ph2C(OH)CH2+.
Which of
the two groups migrate preferentially from the migration origin, i.e., the
relative migratory aptitude depends on the electron donating ability of the
groups, since the rearrangement involves movement of the migrating group with
its bonding electrons to an electron deficient centre. The migrating tendency
of a group may sometimes depends on: (i) its position in the most stable
conformation of the molecule and (ii) whether the group that does not migrate
is better at stabilizing the positive charge develop on the migration origin.
Hence, there is no clear answer in so far as migrating tendencies are
concerned. In general the relative is of migration is found to be:
p-MeOC6H4- >
p-MeC6H4- >
C6H5- > p-ClC6H4-
> o-MeO-C6H4-
> H > R
The
migratory aptitude of hydrogen is often unpredictable. In some cases migration
of hydrogen is preferred to that of aryl and in other cases migration of alkyl
is preferred to that of hydrogen.
Also
Aryl group migrates more readily than alkyl group because the former forms more
stable bridged intermediate. The migratory aptitude of an o-aryl group is less than that of m-aryl or p-aryl group
because of steric hindrance.
However,
there are reactions in which mere electron donating ability does not decide
which one will migrate. It has been found that a group in 'anti' or 'trans'
position with respect to the leaving group, H2O, in the more stable
conformation of the protonated substrate migrates preferentially.
Since
migration of a group occurs to the planner carbenium ion via bridged
intermediate in this rearrangement, the migrating group has no scope to undergo
inversion of configuration; thus its configuration is retained. Perhaps the
detachment of H2O and migration of the group of more or less
simultaneously and the inversion of configuration of the migration terminus is
observed.
When
optically active (S)-2-methyl-1, 2- butanediol is subjected to the pinacol
rearrangement, a racemic product is obtained. This unsymmetrical diol undergoes
protonation on the –OH group at C-2 rather than on the –OH group at C-1 to form
the more stable carbocation EtMeCCH2OH. The carbocation then
undergoes a 1, 2-hydride shift to yield, after proton loss, 2-methylbutanal.
Since the migration of the hydride ion is relatively slow, the carbocation gets
enough time to rotate. As a result of this, a racemic product is obtained.
The
rearrangement is stereospecific and involves migration of the group which is stereochemically
anti to the departing –+OH2 group: The rearrangement
occurs in an anti-manner and a C—C σ-bond may also play the role of migrating
group if there is no alkyl and aryl group with right geometry at the migration
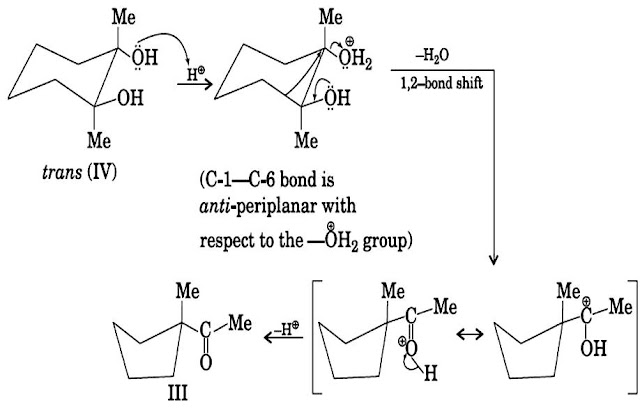
origin. For example, cis-1, 2-dimethylcyclohexane-1, 2-diol(I) undergoes
acid-catalyzed pincol rearrangement to give, two products as follows
When the
axial –OH-group leaves, the axial methyl group on the adjacent carbon migrates to
give 2, 2-dimethylcyclohexanone (II). However, when the equatorial –OH leaves,
bond migration occurs to yield 1-acetyl-1-methylcyclopentane (III). Because of
intramolecular hydrogen bonding, the trans-isomer (IV) exists almost entirely in
the form in which the two –OH groups are equatorial. So, it reacts through this
conformational isomer to yield the ring-contracted product III.
The
pinacol-pinacolone rearrangement is an important synthetic tool in organic
chemistry, particularly for the preparation of ketones from readily available
starting materials. It's also a valuable reaction for mechanistic studies due
to its well-defined mechanism and intermediates.
Reference
1) Jonathan Clayden, Nick Greeves, Stuart Warren, organic chemistry book second edition.




















Comments
Post a Comment