Characteristics of Photoelectric Effect
When
a beam of light (in some cases visible light) is incident on a metal plate
preferably of alkali and alkaline earth metals, some electrons are emitted. An
experiment design is shown here when a beam of light is getting incident on the
metal surface (P) in an evacuated tube, the emitted electrons from the metal
plate are attracted to the positively charged plate (Q). Thus a current flow
through the circuit and it can be detected by the galvanometer (G). This photoelectricity follows the following characteristic features supported by the
experimental facts.
a) Dependence of
photoelectric current on the intensity of incident light: The total photoelectric current (i.e. the
total number of electrons emitted from a given surface) is proportional to the
intensity of the incident beam of a particular frequency. Intensity of a beam
of a particular frequency is measured by the number of photons getting incident per unit area of the surface per second. Thus it indicates, the greater the number
of incident photons of a particular frequency, the greater is the number of
emitted electrons and hence the more current is produced.
b) Threshold frequency
of the incident light to initiate the photoelectric effect: For a particular metal surface, the
incident beam should have a minimum frequency below which there will be no
emission of electrons. This minimum frequency required to start the phenomenon
is called the threshold frequency of that particular metal. If the threshold
frequency is not attended, whatever may intensity of the incident beam there
will be no photoelectric current.
c) Dependence of energy
of the emitted electrons on the frequency of incident beam: The maximum velocity, or the maximum
kinetic energy of the emitted electrons for a particular metal plate is
completely independent of the intensity of the incident beam, but the maximum
energy of the electrons is dependent on the frequency of the incident beam.
Thus the maximum energy of the electron is only dependent on the frequency of
the incident beam and nature of the metal.
The
above facts can be illustrated experimentally. In the figure if the plate (Q)
is made negative with respect to metal surface (p) then the emitted electrons
will experience a retarding potential to reach the collector plate (Q). The
electrons which will have only sufficient kinetic energy to overcome the
retarding potential will only be able to reach the plate. Thus, if the retarding
potential is increased gradually for a particular incident beam of a certain
frequency, then at a certain potential it will be found that no emitted
electron will be able to reach the collector plate. This minimum required
retarding potential to stop the photoelectric current is called the stopping
potential (Vs).
½
mumax2 = eVs or umax = √(2eVs/m)
Here
it is noteworthy that all the electrons emitted are not having the same
velocity. The electrons emitted just from the surface will have the maximum
energy while the electrons coming from the bulk will have the lower energy.
Thus the energy of the electrons may vary, but the maximum energy which can be
associated with the emitted electrons is fixed for a certain frequency of the
beam incident on a particular metal surface. Thus, stopping potential is found
to increase linearly with the increase of frequency of the beam while it is
independent of the intensity of the beam.
Now, the potential of the collector plate is moved towards the positive direction
with respect to plate (P) and the current goes to increase. But after
certain positive value of the potential on Q, there will be no further increase
of current. This is called saturation current. It means that all the liberated
electrons having energy, minimum (i.e. umin) to maximum (i.e. umax)
have been able to reach the collector plate.
Einstein’s Theory of
Photoelectric Effect
According
to Einstein's theory, when a photon strikes an electron, either the whole
energy of the photon or no energy of the photon will be transferred to the
electron. Thus it follows the principle, all or none. If a photon gets incident
on the metal surface and the energy transfer process occurs then a proportion
of the energy will be used to make the electron free from the binding forces of
the metal and the residual energy will be utilized to import the kinetic energy
to the released or free electron.
If
the target electron lies below the surface, it will require some additional
amount of energy to reach the surface, but if the target electron originates on
the surface, it will not require this additional energy. Thus the energy of the
incident photon will be utilized in three different successive steps:
(i)
bringing the target electron to the surface from the binding forces within the
metal,
(ii)
releasing the electron from the surface, and
(iii)
imparting the kinetic energy to the released electron of zero velocity on the
surface.
This
is why, for a particular type of photons of hν, the electron which originates
on the surface will have maximum kinetic energy because it does not require any
additional energy as required by the bulk electron. Under such circumstances,
the electron on the surface will required the minimum amount of energy (ω) to
overcome the binding forces to get released. This minimum energy, ω represents
the work function of the metal and it is expressed in terms of threshold
frequency (νo) as ω = hνo. It indicates that the energy
of the incident photon is less than ω or hνo, there will be no
emission of photoelectron.
In
view of the above fact, for the incident photon of hν, according to the
principle of conservation of energy, it can be written as follows:
½
mumax2 + ω = hν
½
mumax2 = hν - hνo
eVs
= h(ν – νo)
This
is the Einstein's equation. The above equation can explain all the observations
which did not get any support from the idea of classical electromagnetic
theory. It implies the following conclusions:
Concept of threshold
frequency or work function:
If the frequency of the incident photon is less than the threshold frequency νo
(in terms of energy, hv < work function, ω), there will be no photoelectric
emission, regardless of the intensity (i.e. the number of photons getting
incident on the metal surface per unit area per second).
Relationship between
stopping potential and frequency of the incident beam: The maximum kinetic energy and
consequently the stopping voltage (Vs) are independent of the
intensity of the incident photon but linear relationship with the frequency of
the incident photon. This aspect was verified experimentally by Millikan.
According to the Einstein's equation we have:
Vs
= hν/e – νo/e
Thus
the plot of Vs against ν gives straight line whose slope is given by h/e and
the magnitude of the intercept is given by hνo/e. From the slope, h
can be obtained from the known value of charge of the electron. Millikan
determined the value of Planck constant in this method and it was in good agreement with the result obtained from other sources this verification
definitely gives a sound support to the validity of Einstein's equation.
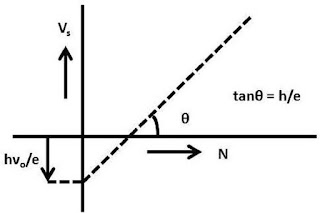 |
| Variation of stopping voltage for the emitted photoelectrons with the frequency of the incident radiation |
Work function and
ionization potential:
The
electrons in a metal are having some potential energy. The potential energy of
an electron for a particular metal depends on its position. The electrons at
the outermost shell of the atoms deciding at the surface are having the minimum
binding energy. On the other hand, for the same type of electrons within the
bulk, the binding energy is higher. This is why; the most loosely bound
electrons (i.e. At the outermost shell) of the atoms at the surface require the
minimum amount of energy to get released from the binding forces. The minimum
required energy is called the work function of the metal. In the case of
ionization potential, it gives the required minimum amount of energy to release
the most loosely bound electron from the metal atom in isolated and gaseous
condition. Thus both the process deal with the required minimum amount of
energy to knockout the most loosely bound electron. But in the case of
photoelectric effect, the metal atom is in the solid state at the exposed
surface while in the case of ionization potential, the metal atom is in gaseous
or isolated condition.
|
Metal |
λo
(nm) (threshold) |
Work
function (in eV) |
Ionisation
(1st) potential (eV) |
|
Na |
500 |
2.50 |
5.12 |
|
K |
550 |
2.26 |
4.32 |
|
Cs |
660 |
1.87 |
3.88 |
|
Cu |
290 |
4.30 |
7.70 |
|
Ag |
261 |
4.73 |
7.54 |
|
Zn |
359 |
3.44 |
9.37 |
|
Fe |
262 |
4.71 |
7.83 |
|
W |
261 |
4.73 |
|
|
Pt |
196.2 |
6.30 |
|
Reference
1) Concise inorganic chemistry by J. D. Lee.
2) Inorganic Chemistry by James E. Huheey, Ellen A Keither, Richard L. Keither, Okhil K. Medhi.

Comments
Post a Comment