A group
of organic species having a positively charged carbon atom bearing only six
bonded electrons is called carbenium ion or carbocation. For example, CH3+,
CH3CH2+, etc. are considered as carbenium ions.
The
electronic configuration of methyl cation is as follows:
Heterolytic
cleavage of a bond gives rise to a carbenium ion. Heterolysis is carried out by
several methods:
1) Direct ionisation method: In this
method a group leaves the substrate with a pair of electrons. In this case a
highly polar solvent medium having high dielectric constant is required. Since the reaction is reversible, the anion formed needs to be removed. Ag+,
Zn2+, Hg2+, etc. can readily do the job.
CH3Br
+ Ag+ ⇋
AgBr + CH3+
2) Addition of acids to multiple bonds:
Acids may be used in presence of alkenes and alkynes to form carbenium ions.
Acids in this case may be protic acid or Lewis acid. Addition of cations to a
multiple bond also generates a carbenium ion.
3) Abstraction of an atom or group using
protic or Lewis acids: In this case a lone pair of electrons of the
substrate co-ordinates either with the proton or with the Lewis acid and then
decomposes to a carbenium ion.
4) Decomposition reaction: In such cases organic species decomposes to
produce a carbenium ion. Example: diazonium ions decompose to form carbenium
ions.
CH3-N≡N
⇋ CH3+
+ N2
5) Rearrangement of carbenium ion: In this
case a less stable carbenium ion rearranges itself to form a more stable
carbenium ion. Neopentyl carbenium ions rearranges readily.
Carbenium
ions are short-lived species. They can accept electrons and hence may be looked
upon as Lewis acid. They undergo four types of reaction:
1) Combination with an anion: In this case
one often gets a stable product. Thus, When HCl is added to ethylene, first
ethyl cation is formed which combines with Cl- ion to form ethyl
chloride.
2) Elimination of a proton: In this case a
stable unsaturated compound is formed; for instance, isopropyl cation gives up
a proton to form propylene.
3) Addition to a multiple bond: In this
case another cation is formed which then produces a stable compound by another
reaction.
4) Rearrangement reaction: It has already
been discussed how carbenium ions rearrange themselves.
The
three bond axes of a cationic carbon have been found to have a plannar
triogonal orientation like those of boron in BF3 molecule. So the C
atom carrying the positive charge is considered to be sp3 hybridised
in a carbenium ion; one of its p A.O.s remains vacant.
Stability
of a carbenium ion may be ascertained by considering:
a)
Inductive effect,
b)
Conjugation effect,
c)
Hyperconjugation or resonance effect,
d)
Solvation effect,
e)
Steric effect.
The
stability of alkylcarbenium ions and radicals is well explained by considering
inductive and hyperconjugative effects and the order are found to be:
(CH3)3C+ >
(CH3)2CH+ > CH3-CH2+
> CH3+
(CH3)3Ċ
> (CH3)2CḢ > CH3-CH2̇
> CH3̇
Several effects need to be considered for explaining the given order:
Hyperconjugation
effect: We know that the more the number of contributing structures of
comparable energy in a resonance hybrid, the greater is the stability. In
tert-butyl cation, there are nine α C-H σ-bonds and hence its resonance hybrid
consists of ten resonating forms, nine of which are uncharged structures; we
shall have six similar resonating forms in isopropyl cation, Three such in
ethyl cation and none in methyl cation. Therefore, the order of stability of
the carbenium ions is as given in the question.
Inductive
effect: This can be explained by +I effect of the methyl groups. Thus, due to
the +I effect of the three methyl groups attached to the positively charged
carbon atom of tert-butyl cation, its charge is neutralized to a greater extent
than that of isopropyl cation which possesses only two methyl groups for such
an effect; this charge neutralization effect is still days in the case of ethyl
cation as it contains only one methyl group and it is least in the methyl
cation because it does not have any electron releasing group attached to the
positively charged carbon atom.
The
order for the stability of the radicals can be explained by hyperconjugative
effect.
(CH3)3Ċ
has nine resonating forms without odd electron in its resonance hybrid, (CH3)2CḢ
has six such structures, CH3CH2̇ possesses only three
structures without odd electron, and methyl radical has none. Therefore, the
order of contributing structures in the resonance hybrid of the radicals is
The
stabilities of allyl and benzyl cations are found to be high; conjugative
effect can explain their stability:
CH2=CH-CH2+
⇋ +CH2-CH=CH2
Resonance: The
resonance hybrid of allyl cation consists of two equivalent resonating
structures and hence they have equal contributions to the hybrid. Thus, it is a
stable cation.
Resonance
is a major factor influencing the stability of carbenium ions. When the positive
carbon of a carbenium ion is α to a double bond, effective charge delocalisation
with consequent stabilization occurs in allyl and benzyl cations, for example, are
found to be highly stabilized by resonance.
Steric effect: Steric
effect causes an increase in stability of tertiary carbenium ions having bulky alkyl
groups. For example, the substituents in triisopropyl cation (having planar
arrangement with 120oC angles) are far apart from each other and so
there is no steric interference among them. However, if this carbenium ion is
added to a nucleophile, then a change of hybridization of the central
carbon atom from sp2 (trigonal) to sp3 (tetrahedral)
takes place and the bulky isopropyl groups are pushed together. This will
result in a steric strain (B strain) in the product molecule. Because of this,
the carbenium ion is much reluctant to react with a nucleophile, that is, its
stability is enhanced due to steric reason.
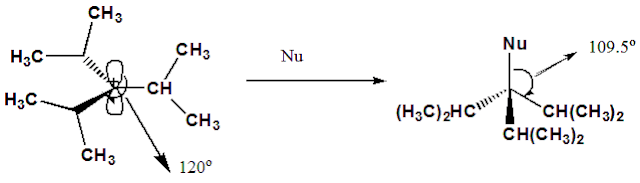 |
| Lesser steric crowding to larger steric crowding |
Solvent effect: carbenium ions are species with a positively charged carbon atom. Their stability in
solvents depends on the nature of the solvent. In polar solvents such as water
or alcohol, carbenium ions are more stable because these solvents can stabilize
the positive charge through interactions with their negative ends. Non-polar
solvents like hexane or benzene, however, don't provide such stabilization
making carbenium ions less stable in these environments.
In a
nutshell, the following rules are to be remembered for comparing of carbenium
ions (carbenium ions).
1) The
more the +I effect on the carbon atom bearing the positive charge, the more
stable is the carbenium ion.
2) The
more the –I effect on the carbon atom possessing the positive charge, the less
stable is the carbenium ion.
3) The
more the delocalisation of positive charge through conjugation, the more stable
is the ion.
4) The
more polar the solvent, the more stable is the carbenium ion through solvation,
provided there is no chemical reaction between them.
Carbenium
ions discussed so far are called classical carbenium ions which contain two-electron two-centre bonds. There is a type of carbenium ion which the positive
charge does not remain on a single carbon atom but spreads over atleast three
atoms and those three atoms form a cyclic cation and are thus called bridged
carbenium ions or non-classical carbocations. β-phenethyl cation is an example of
non-classical carbenium ion:
Bridged
cations with delocalisation bonding σ electrons are called non-classical
carbenium ions which contain two-electron three centre bonds. These are now
called carbonium ions.
Non-classical
norbornyl cation forms as an intermediate when exo- and endo-norbornyl
brosylates (brosyl group is p-BrC6H4SO2) are separately subjected to acetolysis
in the acetic acid medium containing potassium acetate. Norbornyl cation then
gives racemic norbornyl acetate though the two substrates, the exo- and
endo-forms, are diastereomeric. However, the former one reacts much faster than
the latter and direct non-classical carbenium ions formation by the neighbouring
group participation is said to be the cause for the very fast rate of the
reaction. The endo-isomer, under the same set of reaction conditions, undergoes
the reaction but slowly. It is supposed that a classical carbenium ion forms
first which then changes slowly to the non-classical carbenium ion before the
reaction with acetic acid and potassium acetate.
 |
| Norbornyl carbenium ion |
Bridge-head
carbocations or carbenium ions are different from bridged or non-classical
carbenium ions. Carbon atoms at the junction of a bridged ring system are
called bridged-head carbons. When a bridge-head carbon bears a positive charge,
it is called a bridge-head carbenium ions as shown below:
Bridge-head
carbenium ions are highly unstable since the cationic carbon cannot assume its
usual planner trigonal orientation of its bonds. However, (b) is less strained
and hence less unstable than (a); perhaps a six membered ring being more stable
than a five membered ring, (b) is more stable than (a).
1) Jonathan Clayden, Nick Greeves, Stuart Warren, organic chemistry book second edition.











Comments
Post a Comment